
The third part of the HUN-VE series of studies was published at the end of July. This is a study that systematically assesses the effectiveness of coronavirus vaccines used in Hungary and is unique in that it has government support as well as high professional quality. HUN-VE 3 tested vaccine effectiveness against the then dominant Delta variant during last autumn's pandemic wave and found that although the effectiveness of all vaccines declines over time after vaccination, the Chinese Sinopharm vaccine offers the lowest protection in almost all respects. According to the authors, this is the first study to investigate the effectiveness of Sinopharm's booster vaccine, particularly in comparison with mRNA boosters. The study was published more than a month ago, but its results have not been communicated anywhere.
One of the weakest points of managing the Covid-19 pandemic in Hungary has been communication: the way data is communicated, the amount of it available and the transparency of the whole process leave much to be desired. This is particularly true for the issue of coronavirus vaccines. However, the latter has taken a turn with the launch of a series of studies, HUN-VE, to assess the effectiveness of vaccines used in Hungary. It is unique in that the then Ministry of Human Resources (Emmi) was involved, and its authors include Cecília Müller, the chief national medical officer and Miklós Kásler, who was then Minister of Human Resources.
We see more and more clearly
The results of the first HUN-VE were published in a prestigious international journal, Clinical Microbiology and Infection, in November last year. As we wrote in our article at the time, this was the first Hungarian study to show the protection of vaccines against infection and mortality in Hungary, broken down by vaccine type. It found that all five vaccines widely used in Hungary provide very effective protection. However, there was a serious limitation: the data were only from the period up to June last year, so they did not include the period after which the effectiveness of vaccines began to decline as time passed after the vaccination. This meant that it was not really possible to compare the performance of the different vaccines, as it was foreseeable that the differences between them began to emerge only after the period under study.

This was done by another Hungarian study though, the results of which were published in February this year. For the first time it reported data on how the effectiveness of the vaccines used in Hungary declined over time against infections. The authors found that although all vaccines showed a decline in effectiveness, the Sinopharm, Sputnik V and AstraZeneca vaccines showed the most significant decline. The two mRNA-based vaccines, Pfizer-BioNTech and Moderna, held up best.
Then, in June, HUN-VE 2 was published in the equally reputable international journal Frontiers in Immunology. It looked at the end of 2021 and the beginning of 2022 and it did show a decrease in effectiveness. However, one of the strengths of the first HUN-VE, the reporting of results by vaccine type, was not included in this second study.
This effectiveness by vaccine type was demonstrated by HUN-VE 3, the results of which were published on 22 July, also in Frontiers in Immunology (and before that as a preprint in April). This time, however, there was no official communication that a large domestic study was reported on in an international journal – even though the results of the first HUN-VE were published in November on the official epidemiological information page, and a governmental statement was also issued on HUN-VE 2.

Sinopharm's vaccine was less successful
The study looked at the effectiveness of two and three doses of vaccines in preventing infection, hospitalisation, and death in last autumn's Delta wave, and the durability of this protective effect over time. This was done by comparing the morbidity, hospitalisation, and mortality data of vaccinated people with similar data from an unvaccinated control group. The study analysed data from more than eight million participants aged 18-100 years. Statistical methods were used to adjust for differences due to gender, age, underlying diseases, and time of vaccination. This is important because different vaccines were given to subjects of different age, sex, and those suffering from various chronic diseases.
Although the first HUN-VE concluded that, based on the data at the time, all the vaccines used in the country were highly or very highly effective in protecting against infection and mortality, it was already clear from the results that the Chinese vaccine had the lowest overall protection against infection, protecting only 43.1 percent of the most vulnerable age group (those over 85) against infection, even in the period under examination, which was close to the time of vaccination.
This picture was further nuanced by HUN-VE 3, as vaccine effectiveness declined and booster vaccines became increasingly important, the Sinopharm vaccine stood out even more.
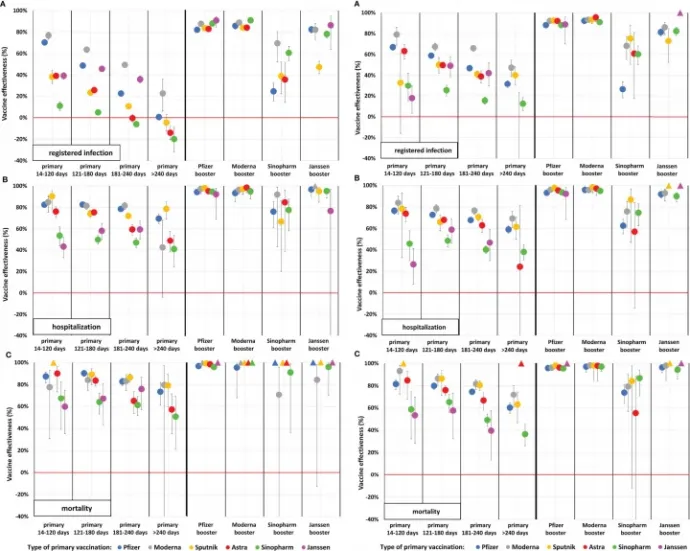
The lessons learned from the study are well summarised in the following graphs, which show vaccine effectiveness by different outcomes following infection, age groups, vaccine types and vaccine combinations. On the left is the effectiveness for 16–64-year-olds and on the right for 65-100-year-olds, shown from top to bottom, against infection, hospitalisation, and mortality. The left half of each graph shows the effectiveness of the primary vaccination (i.e. one vaccination for the Janssen vaccine, two for all others) with increasing the time passed since vaccination, and the right half shows the protection of the different types of booster vaccines (Sputnik V and AstraZeneca vaccines were not given in significant quantities as booster vaccines and are therefore not shown.) The colours indicate the vaccine used for the basic immunisation.
As can be seen, protection against infection declined the most, but even against the Delta variant, this was still brought back up by most booster vaccines (we know from elsewhere that it was somewhat different against the Omicron variant). Protection against the more severe outcomes was more durable and better, but the third dose also raised it significantly. The effectiveness of Sinopharm's vaccine (marked in green) was consistently among the lowest in all categories and stood out markedly when given as both a baseline and as a booster vaccine (where there is a triangle instead of a dot, there was not enough data to evaluate because few people received the vaccine combination):
Let's look at all this in a bit more detail.
In the Delta wave, the effectiveness of the primary vaccination against infection was initially (14-120 days after the baseline vaccination) 11-79 percent, depending on the type of vaccine, which dropped to 0-40 percent six months after the second dose – this was a foregone conclusion, we know that the vaccines are less and less effective in preventing infection.
However, the third vaccine still gave a significant boost against infection with Delta but here the differences between the different vaccines were already striking. For those aged 65 and over, a booster dose of Pfizer-BioNTech's vaccine boosted immunity to 88-93 percent (depending on the vaccine used for primary vaccination), Moderna's vaccine to 92-96 percent, and Janssen's to 73-86 percent. However, for those who received a booster vaccine from Sinopharm, protection against infection was only 27-75 percent.
As expected, the next category, protection from hospitalisation, is already higher for most vaccines, but the gap between the different types is widening here too. Pfizer-BioNTech: 77 (83 for under 65s) for people aged 65 and over, Moderna: 84 (85), Sputnik V: 78 (90), AstraZeneca: 74 (76), while Sinopharm: 46 (54), Janssen: 26 (43) percent within 120 days of the primary vaccination. Here the decline in effectiveness was also more modest, with most vaccine types bringing the booster vaccine close to 100 percent. Except for Sinopharm's vaccine, which proved less effective as a booster vaccine in this regard as well: depending on the type of baseline immunisation, it gave between 57 and 92 percent protection – i.e., even the best case did not match the worst results of all other vaccines.
Finally, the protection against mortality, which is of course the main function of vaccines, was as follows. Pfizer-BioNTech: 82 above the age of 65 (87 under the age of 65), Moderna: 93 (78), Sputnik V: 86 (89), AstraZeneca: 85 (90), but Sinopharm: 59 (67), Janssen: 54 (60) percent immediately after the primary vaccination. Here too, there was a modest decrease over time, and the booster vaccine also produced close to 100 percent immunity. Except, again, for Sinopharm's vaccine: under 65 years, this was still in order, but over 65 years it only increased immunity to 55-87 percent.
According to the authors, HUN-VE 3 is the first study to provide clear and comparable results on effectiveness against severe disease in the Delta wave for six different vaccine types: "The HUN-VE 3 study demonstrated waning VE with all vaccine types for all examined outcomes during the Delta wave and confirmed the outstanding benefit of booster vaccination with the mRNA or Janssen vaccines," the authors write in the abstract at the beginning of the study, conspicuously omitting Sinopharm's vaccine from the list. However, they later added:
"On the other hand, our study showed less improvement in adjusted vaccine effectiveness after the Sinopharm booster against SARS-CoV-2 infection and Covid-19 related hospitalization and death both in younger and older age groups."
The authors of HUN-VE 3 also note that although the Janssen vaccine showed inferior effectiveness as basic immunisation in some outcomes, it produced strong protection similar to that of mRNA vaccines when given as a booster vaccine for any primary vaccination.
The finding of HUN-VE 3 that the Sinopharm vaccine was the weakest booster vaccine is also noteworthy because the official Hungarian recommendation for the third vaccine explicitly recommended its use: after Pfizer-BioNTech and Moderna basic immunisation, the Sinopharm vaccine was recommended as the first choice. Independent experts already made alternative proposals then, based on the information available at the time, that would have avoided the use of the Sinopharm booster, at least in the elderly and high-risk groups, but their proposal was not even considered by the authorities.
A good starting point for the future
The comparison of the effectiveness of different vaccines is not important so that one political side or the other can tout the results. On the contrary, it is precisely the extreme politicisation of vaccine procurement that makes it impossible, or at least very difficult, to carry out the process that is at the heart of how science works: to constantly evaluate and, if necessary, correct the strategy based on new data and fine-tune it in the light of new results (where necessary, since, as we have seen above, the Russian Sputnik V, for example, has performed well).
In an ideal world, a government would have no problem saying that, although it may have been justified and useful to introduce certain types of vaccines under the circumstances (leaving aside the questionable way in which they were obtained and introduced), more information has since been gathered or other, better vaccines have become available in sufficient quantities to warrant moving in a different direction. However, when vaccine procurement becomes a political battleground, it is more difficult to fine-tune it politically on the fly, as we have seen with the self-contradictory communication of the government's subsequent return to the EU procurement of the Pfizer-BioNTech vaccine. In any case, in the field of vaccines, it is precisely the HUN-VE trial series, which is also supported by the government, that can provide purely professional grounds for future directions in epidemic management.
For more quick, accurate and impartial news from Hungary, subscribe to the Telex English newsletter!
The translation of this article was made possible by our cooperation with the Heinrich Böll Foundation.