The distributor of the banned "lung vitamin" has emerged behind yet another suspicious dietary supplement

- On July 1st 2022, Hungary's National Food Chain Safety Office announced that the vitamin supplement Air7, formerly advertised under the slogan "vitamin for the lungs", is being withdrawn from the market due to misleading advertising practices. The Hungarian Competition Authority wrote to Telex that it had also launched an investigation and temporarily banned advertising of the product.
- Meanwhile, a dietary supplement called Herbapirin, also produced by the distributor of Air7, has appeared on the market, and the product, which was initially advertised as an "herbal pain reliever", faces very similar problems: the claims made about it are not scientifically substantiated and are highly questionable from a legal point of view.
- In response to our inquiries, both authorities have indicated that they are investigating the matter, and the Association of Hungarian Dietary Supplement Manufacturers and Distributors have also contacted the company, which amended its publicized claims regarding the product following our inquiry.
- Behind the scenes of Air7 and Herbapirin stands a certain Viktor Koltai. On paper, he has no connection with either the former or the current distributor, but his traces can be found in a few places with both products, and he is reported to have previously negotiated with business partners about Air7. Since then, the company in question has been liquidated and the partners have been waiting for their money to no avail. Meanwhile, Koltai has been busy inventing wildlife alarms.
"Researchers make a new breakthrough: an herbal pain reliever", proclaimed Hirado.hu, the flagship news service of Hungary's public media, in an article published on June 8th 2022. News of Herbapirin Rapid swept through half of the Hungarian media: following the aforementioned article a few other newspapers reported on the supplement, and a couple of days later they were followed by several other sites, from Világgazdaság to Origo and Magyar Nemzet. All the articles are essentially identical word for word, i.e. said newspapers likely reproduced the distributor's press release without any changes (incidentally, Telex did not receive such a release).
The articles made several remarkable claims about what they called the "first herbal pain reliever":
- "the result of decades of research" and "decades of work by Hungarian pharmacists and herbal specialists";
- "revolutionizes pain relief for those who wish to avoid taking synthetic drugs";
- "The herb's secret is that it is very similar to one of the most popular painkillers, aspirin, as it is a natural source of salicylate. White willow is slower-acting than aspirin, but its effects last longer and it has fewer – if any – side effects. White willow bark is effective in relieving headaches, mental stress, and acute muscle pain, but it can also be used to treat other chronic pains such as back and neck pain";
- "The carefully selected, natural active ingredients (such as rosemary, turmeric or willow bark) combine to help relieve pain and the inflammatory processes that cause it, whether of neurological or arthritic nature";
- “It is perhaps surprising that an herbal medicine can be as effective as a traditional painkiller. In fact, it is much easier on the body and it has no side effects.”
According to its website, besides salicin (willow bark extract), Herbapirin Rapid contains rosemary, turmeric, and pine bark extracts. The prescribed dosage is one tablet per day, and the recommended retail price of a 20-tablet pack is HUF 2890. Although their own webshop was reporting a temporary stock shortage at the time of writing, several other webshops had it in stock, with a price range of HUF 2800-3000 per box.
If reading the above gives you the feeling that all of this sounds almost too good to be true, then your instincts are unfortunately spot on: for one thing, these claims are not scientifically substantiated, and even if they were, it is still legally problematic to promote a dietary supplement with such claims.
Scientifically unsubstantiated
First, let's take a look at the product itself and how the claims of its efficacy compare with what the science currently says. We'll start with the ingredient that is touted as the most important: willow bark extract.
"Willow bark does have analgesic properties, but you would need to consume a much higher dose than the one in this product," said pharmacist, herbal researcher, and founding author of the professional blog Pirulakalauz, Dezső Csupor.
The single tablet to be consumed per day contains 200 milligrams of willow bark extract, of which the active ingredient, salicin, is 30 milligrams. "Compare the 30 milligrams of salicin to the related compound found in aspirin, acetylsalicylic acid, which has a dose of 500 milligrams. The dosage of salicin needs to be higher than that because it is a larger molecule. Therefore, if you want to achieve the same potency, you need considerably more in terms of mass, about 800 milligrams. Even then, the effect would not be the same, because acetylsalicylic acid is faster acting," said Csupor.
"Willow bark is a slower-acting substance, and the supplement has about an order of magnitude less of it than the dose required in order to have any effect at all."
In light of its slower mechanism of action, it is particularly interesting that the word rapid has been included in the name of the product.
But let's continue on with the other ingredients.
Rosemary
According to the press release quoted in the above articles, "the extract of Rosmarini aetheroleum, or rosemary oil, may be beneficial in the treatment of muscle and joint pain."
However, according to Dezső Csupor, rosemary when taken orally, i.e. in tablet form, has no analgesic effect at all: "I suppose it was put in because rosemary is used externally, as a skin rub, to relieve joint pain. But if you eat the rosemary extract, it won't have any such effect. It's an antioxidant, sure, but it doesn't relieve pain," he said, adding that from a logical point of view, it is strange to mention essential oils in a pill.
Turmeric
According to the release, "'Curcumae longae extractum', an extract of turmeric, can reduce the formation of inflammatory mediators, thereby having a positive effect on the inflammation process and speeding up its resolution."
According to the herbal researcher, “Turmeric does in fact have an anti-inflammatory effect, but you would need to take about 100 milligrams of its active ingredient, curcumin, to produce any noticeable effect. In comparison, the product contains considerably less than this: 50 milligrams.”
Pine bark
The release reads, "'Pycnogenol' is an extract of pine bark. The active ingredient of pycnogenol may be beneficial for joint pain caused by osteoarthritis and may reduce the need for non-steroidal anti-inflammatory drugs."
According to Csupor: "There is indeed some data that Pycnogenol may be an additive, supplementary agent, but it is not by itself suitable for reducing pain.
Overall, this combination is unlikely to be effective in light of the scientific evidence. Individually, the ingredients are under-dosed and the combination is not complex enough to be effective overall either,"
– the herbal researcher concluded.
So what about the claim that Herbapirin is not only as effective as a medicine but that it is even preferable to take because it has no side effects? "That's not true – I don't think there is any evidence for that. For this particular preparation, I don't think they have any data on whether it has side effects or not, because you would have to have tested it to say that. The fact that these are herbal substances does not mean that the ingredients cannot have side effects, whether taken separately or together. It is a different matter that in this low-dosed form they are unlikely to cause any serious problems, but stated this way is misleading, and it reinforces the misconception that there are bad medications and then there are good herbs – which is not true," said Csupor.

We also asked him about the claim that Herbapirin had been developed by local pharmacists and herbalists over the course of decades. "I headed the Medicinal Plant Division of the Hungarian Pharmaceutical Society for a number of years, and I have not heard of any researchers who have conducted work on this subject for this company, although I know almost everyone in Hungary who does this kind of research. I can't say that it's definitely not true, but I've never heard of such a thing, and if such a thing was going on, I'd probably know about it," replied Csupor.
The fact is, however, that even if all the above claims were completely justified scientifically, it would still not be possible to make such statements about Herbapirin. This is simply due to the fact that Herbapirin is a dietary supplement, which is subject to a very clear regulation: it cannot be claimed that it has healing properties.
Official investigations are underway
The dietary-supplements market is subject to a particular set of regulations. In the past, such products also required authorization, but since Hungary's accession to the EU, according to the applicable EU legislation, this is no longer required: distributors are only required to register with the National Institute of Pharmacy and Nutrition (NIPN). This declaration has to be submitted no later than the day the product is made available on the market.
Initially, NIPN only checks the formal requirements of the declaration and then registers the product. However, as the list available on the download page of the website warns, "the decision does not constitute a manufacturing or marketing authorization, nor does it imply compliance with the product specifications." (Once registered, the authority will also launch a risk assessment.)
These products are therefore not subjected to any substantive testing before they reach the shelves – they are simply registered. But not only are they easier to place on the market, but they are also easier to advertise, as they are not subject to the strict advertizing regulations that apply to medicines. On the other hand, they cannot be said to possess any healing properties. Article 6(2) of Decree No. 37/2004 (IV. 26.) of the Ministry of Health, Welfare, and Family on dietary supplements stipulates:
"In the labeling, presentation, and advertising of a dietary supplement, it is prohibited to attribute to the product a preventive or curative effect or to imply such a property."
With regard to the claims quoted at the beginning of this article and others like them, it may be rightly argued that they may constitute an act prohibited by this decree. We have asked all the relevant authorities: in addition to the NIPN, we have also reached out to the National Food Chain Safety Office (NFCSO), the Hungarian Competition Authority (HCA) and the Ethics Committee of the Association of Hungarian Dietary Supplement Manufacturers and Distributors (AHDSMD) to find out what their position is on the practices of the distributor of Herbapirin, and whether an investigation has been launched or is planned regarding the product.
Not THAT pirin!
The very name of the product may suggest that it is indirectly trying to imply a medicinal effect. After all, tacked on to the plant-related prefix we find the suffix '-pirin', which probably reminds many people of the similarly pronounced '-pyrin'. This latter suffix can be found in the names of several commonly used painkillers. Aspirin, which is related to willow bark extract, is even mentioned in the marketing statement itself, but officially speaking, this doesn't constitute even a coincidental reason as to why the suffix was chosen for the supplement's name. Rather, it's because the salicin it contains is extracted from the white willow (Salix alba), which, according to the website, "is also found in the Pirin Mountains in Bulgaria" (which is indeed the case). Of course, the white willow is also native to Hungary, but you can't expect them to call the product "Herbabükk" [for Hungary's Bükk Mountains].
The NIPN sent a terse response, stating that the newly registered product is still under investigation: "A risk assessment of the product Herbapirin is ongoing at our institute. Based on the results of this assessment, we will take action if necessary."
The other two authorities, however, both replied that, in light of our letter, they would look into the matter. "On the basis of the information provided on the Herbapirin dietary supplement, this request constitutes a matter of public interest. We will carry out an exceptional official inspection, the results of which will be communicated to you at a later stage," NFCSO wrote. The HCA responded similarly:
"No complaints have yet been received by the Hungarian Competition Authority regarding the advertising of the herbal preparation Herbapirin. Nevertheless, your report will be treated as a complaint, and the necessary action will be taken."
In response to our request, AHDSMD, the Association of Hungarian Dietary Supplement Manufacturers and Distributors, sent us a detailed position statement. They described their focus as that of taking action "against distributors of products with unlawful communication and claims of medicinal properties." In cases involving the association's members, this is a simple task because membership is conditional on compliance with their code of ethics. For companies operating outside the association, it is more difficult because they do not have the legal means to do so. Nevertheless, in recent years, several of these cases "have led to substantial changes regarding companies engaging in unlawful communication," hence any flags thrown by the AHDSMD are followed up on by authorities, thus ensuring effective action is taken. Furthermore, in May of 2022, they also entered into cooperation with the Hungarian Advertising Self-Regulatory Board (HASRB), which they expect to take swifter and more focused action in problematic cases.
"At the initiative of a member company, the Ethics Committee launched an investigation into the communication of Herbapirin. The investigation is ongoing, so we cannot yet report on the details, but discussions have started. The distributor of Herbaprin has shown a willingness to cooperate, and positive signs can already be seen on the product's website, where blatantly unlawful messages referring to pain relief and other forms of treatment have been removed. Neither the AHDSMD nor the market surveillance authorities are able to take down the news stories you refer to in your letter that present a product as a scientific breakthrough, but any future communication along these lines can of course be prevented as a result of the action described above."
Subsequent rewriting of the articles
As of the writing of this article, the product website has been careful not to make any scientifically or legally objectionable claims. For example, they don't say that it is the first herbal pain reliever but rather "the first herbal Herbapirin Rapid", which is not something that you can really argue with, even if it doesn't make much sense. As the AHDSMD pointed out, the product was previously described on the website as a pain reliever. Although this wasn't retained in the internet archives, the source of the page, which can be viewed in a browser, still holds a trace of it in the code (click on the image to enlarge):
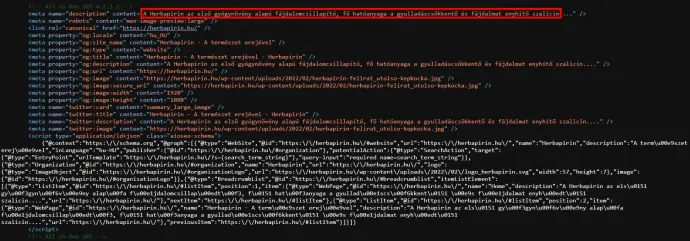
Likewise, the problematic statements found in the promotional articles about the other constituents are still in the code, but are no longer visible when you visit the page.
It is also interesting to note in the response of the AHDSMD that they are only able to take action against future cases of misinformation – there is nothing they can do about the published articles after the fact. In fact, the distributor of Herbapirin itself has done exactly that, apparently asking for corrections. We know this because, naturally, we also asked the company itself, via the email address provided on the website, what their position was on the legal basis for their claims and what scientific evidence they used to make these claims. They did not respond to the latter question (and several others), but they did address the legal concerns: "Thank you for your inquiry. There are ongoing discussions with the supervisory authorities regarding the product. As such, should the supervisory bodies propose any changes to the product or to any claims, we will of course comply immediately." Six days later, they contacted us again:
"We would like to inform you that we have carried out a self-audit in line with your concerns. We have corrected the press releases with legal assistance. Media outlets will continue to replace the content,"
– they wrote, and linked to two articles that had already been rewritten.
It is somewhat odd for a company to have journalists rewrite an article that is a verbatim copy of its own press release weeks later, but it is instructive to compare the before and after. One of the revised articles is that of Hirado.hu, which we also cited: the only claims that remain in it are the ones that can be substantiated – i.e. not much.
The original title read "Researchers make a new breakthrough: an herbal pain reliever", while the revised version reads "Researchers make a new breakthrough: herbs can also be used to fight inflammation." Instead of "pain reliever" and "active ingredient", the text now reads "dietary supplement" and "component". Furthermore, the supplement no longer "has natural anti-inflammatory and pain-relieving effects" but simply "strengthens the body." They took so much legal precaution that they even included some rather unusual and incomprehensible phrases for newspaper articles, such as: "Carefully selected natural active ingredients (e.g. rosemary, turmeric or willow bark) may combine to help combat oxidative stress (claim: 2125, 4007) and painful inflammatory processes (claim: 2030, 4007), whether neurological, muscular or arthritic (claim: 2598, 4014)."
The entire article was shortened as well, and one of the four ingredients, pine bark extract – which was originally claimed to "reduce the need for non-steroidal anti-inflammatory drugs" – is no longer mentioned at all. The following excerpt is typical example. On the left is the original text about how willow bark is so effective, and on the right is the one sentence that survived the revision:

Otherwise, the fact of the retroactive changes is not reflected in the revised texts. At the time of this writing, a search of the original statements revealed that they were still available in several other publications, including Origo, Magyar Nemzet, and Világgazdaság.
A familiar pattern: the banning of Air7
If all this sounds like a familiar story – a dietary supplement implied to have medicinal properties in newspaper articles quoting press releases – your memory serves you well: in early December 2021, we wrote about a vitamin supplement called Air7, whose distributor was doing exactly the same thing. This case is worth recalling in a little more detail not only because of the similarities, but also because, as we will see, there is a direct link between the two products.
At the time, several newspapers reported that a Hungarian "lung vitamin" was included in the treatment of Covid patients at the hospital in Kiskunhalas. There were several problems with the story: the epidemic hospital in Kiskunhalas had been closed for months, but even when it was open, patients were not being given Air7. Nor has there been any evidence to date that it has any particular effect on the lungs. Moreover, not only did the distributor make dubious claims about lung benefits in general, but it specifically suggested that the vitamin supplement could be effective in treating Covid-19.
Although the marketing has apparently been toned down since then – for example, the slogan "vitamin for the lungs" has been replaced by "every breath counts" – Air7 has continued to circulate more or less uninterrupted in Slovakia and the Czech Republic, in addition to Hungary, since the publication of this article. That is, at least until July of 2022, when the NFCSO announced that the product would be withdrawn from the market due to misleading marketing practices, and the distributor would be fined millions of euros. "The product may only be placed back on the market after the information intended for consumers has been corrected," the NFCSO said in its decision.
According to sources, although the decision to ban Air7 has only recently been made, authorities had already been aware of the problems with the vitamin supplement long before. The NIPN had already identified problems in April 2021 during its post-registration risk assessment: according to a letter sent to pharmacies, which Telex has recently obtained, the medicines authority found infringements in the labeling of the product, including "among other things, the name of the product which could be construed as a health claim" and "other misleading claims on the label", such as the phrase "for healthy lungs." According to the NIPN's letter, the Government Office of the Capital of Budapest initiated proceedings against the then distributor Siren7 Ltd., and on 4 May 2021 – six months before our article on the ongoing problems with Air7 – it issued a decision requiring compliance with the law. But despite this, in the letter, the NIPN itself wrote that Air7 remained on the market even though its "marketing was objectionable." (Siren7 Ltd. has since gone into liquidation, but we'll come back to that.)
At the time of this writing, Air7 has not yet been added to the NIPN list of banned products, which continues to reflect the situation at the end of April 2022. An internet search shows that the product is still available in many online shops, as well as on the distributor's own website.
In the meantime, the HCA is also investigating Air7: In response to our query, the competition authority wrote that it had launched proceedings against the product line on August 4th 2021 for "alleged infringement of the prohibition of unfair commercial practices against consumers." The investigation is still ongoing, so no comment was made, but they did say that they had temporarily banned the promotion of Air7 in March of 2022 "to protect consumers," pending the outcome of the investigation. (This was challenged by the distributor in the Municipal Court of Budapest.)
The connection between the new distributor and Herbapirin
The NIPN's records show who has registered a particular dietary supplement, which can be used to identify the distributor. So far, Air7 has been registered by three different companies:
- first in 2020 by a certain Catalase;
- and then in 2021 by Siren7 Ltd. – the distributor at the time of our 2021 article, against whom the aforementioned official procedures have been initiated;
- and then in 2022 by Kalibra Innovative Ltd. – that is to say, a new distributor has emerged behind Air7 in the meantime.
This last development is also important because Kalibra's name can be found on another product, which brings us back to the main topic of this article: it is the same company that registered Herbapirin Rapid.
Neither in this case was Kalibra the first register the product: similarly back in 2022, but a little earlier than in the other case, a certain János Sánta was the original filer (who is probably not the same as the tobacco billionaire János Sánta). He was followed by Kalibra – and this is where the confusion around Herbapirin's distributor starts:
- When we started investigating Herbapirin, there was no reference to the distributor on the site, only an email address, which is unusual given that the product had already been on sale for a while.
- Since then, however, a General Terms and Conditions (GTC) and a Privacy Policy have been posted to the site, and the service provider and data controller in both cases is a company called Herbapirin – it's just that, according to the Opten company database, there is no such company.
- There is also a tax number in the documents, which, according to the company database, belongs to János Sánta, a sole proprietor – however, according to NIPN records, he is no longer the one responsible for the product, but rather Kalibra, the entity that filed the later declarations.
- On the other hand, Kalibra is not mentioned in any way on the Herbapirin website or in the relevant documents.
According to the company database, János Sánta started his business activity in February 2021, and since then the National Tax and Customs Administration (NTCA) has twice taken enforcement action against him. Kalibra was founded in March 2021 under the shared ownership of Martin Bikfalvi and Márton Áron Bikfalvi, who are registered at the same address in Cluj-Napoca, Romania (more on that later).
According to the company database, there is no identification overlap between the former and current distributors of Air7, Siren7 Ltd. and Kalibra Ltd., yet there are several indications that the histories of the two companies coincide at several points. The key link is that
an entrepreneur named Viktor Koltai pops up in both companies, but he is not listed as an owner or director of either company in their paperwork, but several independent sources say that he has regularly negotiated with partners on behalf of Siren7 Ltd. in relation to Air7 and other matters concerning the company.
Not only are these two companies not registered in Koltai's name, but neither are many other businesses. According to the company database, between 2010 and 2014 he was a shareholder in a company called Hungarian Road Paver Ltd., and from 2014 in a company called Life – Automat Ltd., which is currently undergoing forced liquidation. As an individual entrepreneur, he has one entry in the NIPN register of dietary supplements: a dietary supplement capsule called Doctor Breath in 2018, but as of 2020, it is registered under a different name.
Yet there is only one product that has been associated with him publicly: a few years ago, he appeared in the media several times as the inventor of a car-mounted wildlife alarm. The product is still available today under the name Siren7 – i.e. the wildlife alarm invented by Koltai has the same name as the previous distributor of the Air7 "lung vitamin." Meanwhile, on paper, Koltai is not only not affiliated with the latter, but neither with Safety Products Ltd., the distributor of the wildlife alarm.
There are two videos from 2019 on YouTube in which Koltai promotes the "roadkill-prevention system." They show that the man presented as an inventor was primarily into road safety at the time, and he claims to have invented a coating that reduces stopping distance and an illuminated pavement marker for bicycle lanes before the wildlife alarm. Interestingly enough, although the Siren7 wildlife alarm has been on the market since 2019, it was a big sensation in the autumn of 2021, when half of the Hungarian media, that is to say again just Hirado.hu, wrote that the "new Hungarian invention" helps prevent wildlife accidents, and Koltai's name still appears in these articles. He makes no mention of dietary supplements in the 2019 videos, and it seems that it was only later that started to veer off from road safety in this direction.
But it's not just the coincidental naming overlap of Siren7 that connects Viktor Koltai to the Air7 vitamin product. According to Domain.hu's domain registry, Koltai was the domain holder of the Air7.hu website, not only when Siren7 Ltd. was marketing the product, but even when it was Kalibra – that is, until we reached out to Air7's and Herbapirin's customer service email addresses to ask them about this and Koltai's role in the two supplements. The timing could of course be a coincidence, but the fact is that our emails were sent on June 28: there was no response at all from Air7 and the questions sent to the Herbapirin address were ignored. The next day, the domain holder of Air7.hu changed: as of June 29, Kalibra is now on the register:
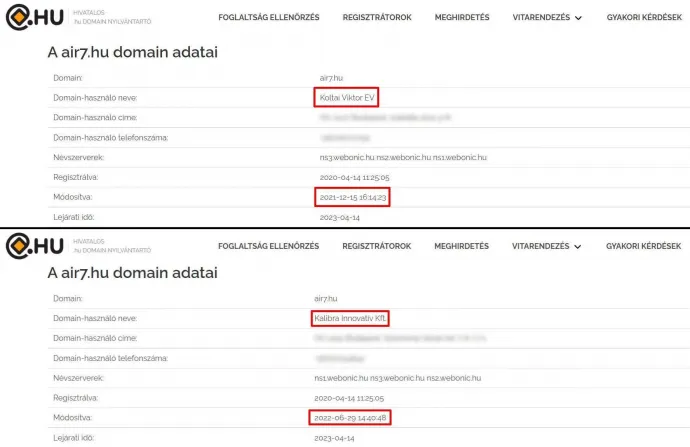
Nothing is listed about the domain holder of Herbapirin.hu except for the fact that it is a physical person (i.e. definitely not Kalibra Ltd.) and that the last time the registration was modified (on December 15 of 2021) was the same time as Air7.hu prior to the current update.
Another similarity is the contact number: the same phone number was given on Air7.hu when Siren7 Ltd. was at the helm as the one that is there now (under Kalibra's direction), but the same phone number can be found next to Safety Products Ltd. on the Siren7 wildlife alarm page. In fact, there's even a fourth page where it can be found: As we mentioned earlier, the Air7 vitamin supplement was first registered with the NIPN by a company called Catalase; an anti-graying capsule is now on the market under this name, and although it is distributed by a different company (by the name of BioGo.hu Ltd.), Catalase's customer service can be reached at the same number. (There is no phone number on the Herbapirin site, just an email address.) Of course, there could be explanations for this correspondence other than a link between the companies, so we called the number and inquired about it. First they promised a call back, but after several attempts since then they no longer answered the phone.
However, the clearest link can be found in the patent register of the Hungarian Intellectual Property Office (HIPO). According to the database, Viktor Koltai's name (and the address associated with him) can be linked to, among other things;
- the Siren7 wildlife alarm (a 2019 trademark);
- the vitamin supplement Air7 (two trademarks in 2020 and one in 2021); and
- the herbal preparation Herbapirin (for which he has filed four trademark applications as of the writing of this article: the first and second in August 2021, and the most recent not long ago, on June 15 2022).
Koltai transferred his three trademarks for Air7 to a Romanian company, Green Splid SRL, in March of 2022. The interesting thing is that it is registered at the same address in Cluj-Napoca as the two owners of the Hungarian Kalibra Innovative Ltd. And although the NIPN database shows that Kalibra is behind Herbapirin, the patents for it are still held by Koltai.
There is a third patent for Air7, but the application is still pending, so there is no protection. On paper, this has nothing to do with Koltai since the applicant is Kalibra itself. (This makes it the only trademark or application in the name of the company, as the other Air7 trademarks are in the name of the Romanian company.) Just as the Air7 trademarks registered by Koltai were transferred to the Romanian company in March 2022, the date of this fourth filing, registered by Kalibra, is also the end of March. Both therefore coincide with the temporary ban imposed by the HCA on the promotion of Air7 pending the outcome of the investigation. (The new filing refers to Air7's new lung-free slogan, "Every breath counts.")

Incidentally, Koltai also has a trademark application for a food supplement for animals under the name "vauu" pending, which was filed in January 2022. Nor does he appear to have left the wildlife-alarm business, as his latest trademark application is dated June 20 2022, for a product called VIONN Go, which its website describes as "the first smart wildlife alarm." This also means that he filed a trademark application for a food supplement and a wildlife alarm in the same week.
So here are two companies that, on paper, have no connection to one another, but have promoted or are promoting their dietary supplements in the same scientifically and legally problematic manner. Further, they are linked by Viktor Koltai, who, according to wildlife-alarm overlaps, domain registrations and patent registrations, has multiple ties to one or the other company, and has reportedly acted on behalf of at least one of them in the past.
We also posed the following questions to the email addresses on the Air7 and Herbapirin websites: Is there a personal or professional connection between Siren7 Ltd. and Kalibra Innovative Ltd.? What is Kalibra's affiliation with Viktor Koltai? Did or does Koltai have any influence on matters relating to the marketing of Air7 or Herbapirin? How is Kalibra related to Green Splid? Is the distributor planning to take over the (pending) Herbapirin trademarks? We received no reply at all from Air7's address. As for Herbapirin, they only responded to the legally problematic advertising practices detailed above – not to these issues.
Kalibra Ltd re-registered Air7 since then. The difference is not clear from the NIPN's list, but at the time of writing only the two previous notification numbers are listed on the Air7 website.
Debts, charges, and liquidation
One other loose end still remains in this story: what ever happened to Siren7 Ltd, the company originally behind Air7? According to Opten's company database, the company, founded in 2020, is in liquidation, having filed for the procedure at the end of November 2021 and started its proceedings in May 2022.
We have spoken to representatives of several companies who have had some kind of business relationship with Siren7 Ltd., which was severed when Siren7 Ltd. did not pay them. According to them, it has become impossible to contact Koltai – even though he has been filing trademark applications in recent weeks.
An update to our December 2021 article on Air7 reveals that although the distributor of the vitamin supplement at the time also listed itself as the domestic distributor of a Swiss-made rapid antibody test, it proved to be misleading: after the publication of that article, the real domestic distributor of the rapid test in question (Lightvision Media Solutions Ltd.) came forward and stated that "Siren7 Ltd. is not involved in any way in the marketing of this product" and that it has no distribution rights to the tests. They ordered tests from them, but as they have not been paid for to date (almost 100 million forints), legal action has been taken.
Reportedly, the debt has yet to be settled, as other partners are waiting for their payment, and an investigation is ongoing (for privacy reasons, the latter has not been confirmed to us by police). And in the course of the liquidation process, large loan claims from foreign firms have emerged, which take precedence over the claims of the Hungarian partners.
The National Tax and Customs Administration last updated the list of individuals with tax debts exceeding HUF 10 million continuously outstanding for 180 days at the end of March – at the time, Viktor Koltai was included in the list.
The market for dietary supplements is becoming increasingly lucrative not only abroad, but also in Hungary, where turnover is estimated to exceed HUF 50 billion a year. It is not clear from the company database what share Air7 and Herbapirin may have carved out of this because the current distributor, Kalibra Ltd., was founded in 2022, so its financial statements are not yet available at the time of writing. Siren7 Kft., which previously distributed Air7, was closed in October 2020, so only that year's data is available, according to which its net sales were 0 and its profit before tax was HUF -8.16 million. On the other hand, the fact that Air7's ads have appeared as TV commercials and on billboards, which cost a pretty penny, and the large debts of Siren7 Ltd. both suggest a larger cash flow.
We also tried reaching out to Viktor Koltai before the publication of this article, but our email bounced back from the address available in the company database, and the phone number listed next to his name in the domain registry is not in service.